Abstract
Introduction: Venetoclax is a selective, oral inhibitor of BCL2, a key regulator of the intrinsic apoptotic pathway. The dose-escalation phase 1 study of venetoclax in patients with relapsed/refractory non-Hodgkin lymphoma (NHL) enrolled 106 patients from June 2011, and the overall response rate across the entire NHL cohort was 44%. The highest response rate (75%) was seen in the 28 patients with mantle cell lymphoma (MCL) (Davids et al., J Clin Oncol. 2017). Here, we report longer-term outcomes for those patients, now with a median of 27 months (range: 0.2 - 59) follow up.
Methods: Venetoclax was administered in dose cohorts ranging from a maximum dose of 300-1200 mg and continued until progressive disease (PD) or unacceptable toxicity; intra-patient dose escalation was allowed. Adverse events (AEs) were assessed by NCI-CTCAE v4.0 and responses were assessed using 2007 Cheson IWG response criteria, utilizing CT scans beginning at week 6. The data cut off for this analysis was June 4th, 2018.
Results: For the 28 patients with MCL, the median age was 72 years (range: 35 - 85). They had received a median of 3 (range: 1 - 7) prior treatments; 5 patients received prior PI3K inhibitor (but no prior ibrutinib). The median time from the preceding treatment to start of venetoclax was 13 months (range: 2 - 148). The median dose of venetoclax was 400 mg/day; 25/28 received at least 400mg/day. Median time on study drug was 11 months (range: 0.2 - 59). Three patients have been on therapy for over 4 years.
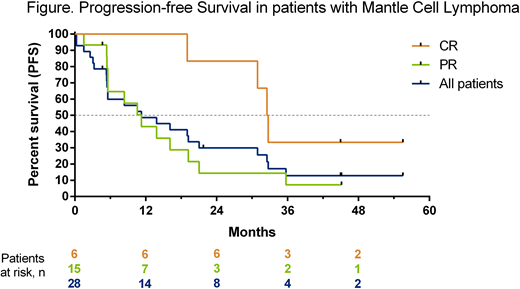
The overall response rate was 75%, with 6 (21%) patients achieving complete remission (CR) and 15 (54%) partial response (PR). The median duration of response was 16 months (95% CI: 4, 30) and median progression free survival was 11 months (95% CI: 5, 21) for all patients (Figure). The 2 year PFS estimate was 30% (95% CI: 14%, 47%) for all patients, 83% (95% CI: 27%, 97%) for patients who achieved CR and 14% (95% CI: 2%, 37%) for patients who achieved PR. One patient who achieved PR proceeded to allogeneic stem cell transplant and remained disease free at the last protocol defined follow-up (24 months after coming off study). Three patients developed progressive disease after receiving venetoclax for more than two years of therapy (time to progression: 31, 33, and 33 months). Two patients with CR continue on study without evidence of progression, currently at 47 and 59 months of venetoclax monotherapy.
The most common (≥25% of patients with MCL) all grade treatment emergent AEs were nausea (57%), diarrhea (50%), fatigue (39%), constipation (29%) and upper respiratory infection (25%). The most common (≥10% of patients with MCL) grade 3/4 AEs were neutropenia (14%), anemia (14%), pneumonia (11%), and thrombocytopenia (11%). Biochemical tumor lysis syndrome (TLS), without accompanying clinical features, was reported in one patient considered high risk for TLS. Specific interventions were not required, and the patient continued on study drug.
Conclusions: Venetoclax monotherapy leads to durable remission in a meaningful proportion of patients with pretreated MCL. Further studies in MCL are currently investigating potential biomarkers for durable response to venetoclax combination regimens, including a Phase 3 randomized study with ibrutinib (SYMPATICO, NCT03112174).
Davids:Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Merck: Consultancy; AbbVie, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy; MEI Pharma: Consultancy, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Surface Oncology: Research Funding. Roberts:Walter and Eliza Hall: Employment, Patents & Royalties: Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone and royalty payments related to venetoclax; AbbVie: Research Funding; Genentech: Research Funding; Janssen: Research Funding. Wierda:Genentech: Research Funding; AbbVie, Inc: Research Funding. Humphrey:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Alter:AbbVie, Inc: Employment, Equity Ownership. Masud:AbbVie, Inc: Employment, Equity Ownership. Buss:Abbvie, Inc: Employment, Equity Ownership. Verdugo:AbbVie, Inc: Employment, Equity Ownership. Seymour:Janssen: Honoraria, Research Funding; Celgene: Consultancy; Genentech Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal